![]() Download the HILT K051537
Download the HILT K051537
![]() Download the HILT510K
Download the HILT510K

Please note any use other than the use as stated in the 510K is considered off label. No statement here is meant to promote off label use.
FDA Approval of HIRO 3.0
HIROTM is a laser device for High Intensity Laser Therapy (HILT), approved by the FDA. It provides safe and quick relief from pain and inflammation, even for deep-seated pathologies.
The Hiro 3.0 has been used successfully with many conditions such as:
- Sport traumas
- Bursitis, Synovitis, Capsulitis, Epicondylitis, and Impingement Syndrome
- Tendinitis and tenosynovitis
- Trauma induced edemas and hematomas
- Post-traumatic or overload induced pathologies
- Abductor syndromes
HIRO 3.0 Specifications:
- High Power pulsed Nd:YAG Laser
- Peak Power: 3,000 W (3,000,000 mW)
- Energy per pulse (max) 350mJ
- Fluence: 1780 mJ/cmq
- Pulse Duration: <120 us
- Standard Handpiece Ø 5mm
- DJD Handpiece Ø 5mm
- Dimensions and weight:
- 11.8" x 27.5" x 30.7"; 88 lbs
- 30 x 70 78 cm; 40 kg
Hand Pieces
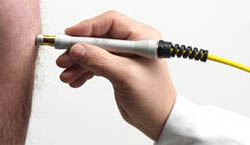
DJD handpiece for regenerative therapy which optimizes the HILT pulse transfer to the tissue. Patented For HIROTM3.0 only.

Standard handpiece for pain therapy featuring a spacer for the correct energy delivery on the treatment volumes.

 Visit us on Facebook!
Visit us on Facebook!


